|
|

12-16 October 2020
★ VIRTUAL EVENT
Delivered in CEST Time Zone
|
|
Sponsored by BSI
Use VIP Code CQ20MTCHBSI to save 25% off* your pass

|
BSI is a trusted, world-class Notified Body and Conformity Assessment Body dedicated to providing rigorous regulatory and quality management reviews and product certifications for medical device manufacturers around the world. Our services include system certification to ISO 13485, CE marking, product testing, and standardization to support your global market access goals. BSI has two Notified Bodies, one in the UK (0086) and one in the Netherlands (2797), both of which have full scope designations to the IVDR and MDR.
Talk Titles
Monday 12 October, 13:30-14:00 CEST
Session: EU Medical Device Regulation, Notified Body Overview and Update from BSI
Speaker: Maddalena Pinsi - Regulatory Manager, Regulatory Services - Notified Body, BSI
Joined for the Q&A by Suzanne Halliday - Regulatory Director & Head of Notified Body, BSI
Tuesday 13 October, 12:15 - 12:45 CEST
Roundtable with BSI: The new UKCA and future UK regulation for Medical Devices and IVDs
Speaker: Jayanth Katta - Senior Regulatory Lead Notified Body, BSI
Tuesday 13 October, 13:30-14:00 CEST
Notified Body Panel Discussion: EU MDR Pain Points
Speaker: Jayanth Katta - Senior Regulatory Lead Notified Body, BSI
Thursday 15 October, 13:30 -13:55 CEST
Session: Initial experiences of applications under the IVDR – A Notified Body's View
Speaker: Erica Conway - Global Head – IVD, BSI
|
|
BRINGING THE LATEST EU MDR, IVDR AND GLOBAL MARKET REGULATORY GUIDANCE TO YOU
Join the European Commission, Competent Authorities, Notified Bodies and industry online for the answers you need to accelerate your path to compliance
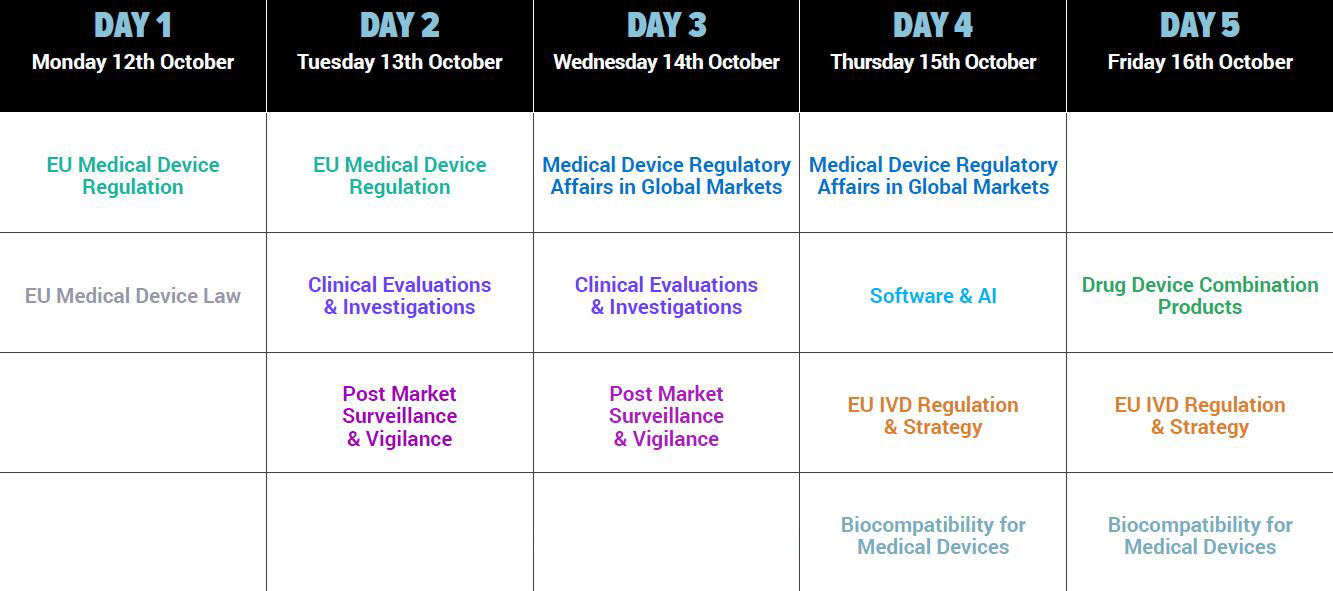
Get the same dedicated coverage of PMS & Vigilance, Clinical Strategies, Combination Products, Software, Law, Biocompatibility and Medical Device Regulatory Project Management from the comfort of your own seat
|
|
|
|
TIME'S RUNNING OUT. JOIN US TO GET YOUR QUESTIONS ANSWERED...
Connect with your fellow industry professionals online this October to benchmark, be inspired and prepare to meet the fast approaching compliance deadlines.
|
|

|
|
*Use VIP code CQ20MTCHBSI to get 25% off*
|
|
*1) Discounts not applicable to supplier/vendor/consultant/solution provider companies. Informa Connect Life Sciences will verify whether you are a supplier/vendor/consultant/solution provider when your registration is processed. 2) Discounts not to be used in conjunction with any other discount. 3) Discount not applicable to existing bookings.